|
View: 5579|Reply: 31
|
Shah Iskandar Tampil Mohon Maaf Jual 'Test Kit' Covid-19 Di IG
[Copy link]
|
|
|
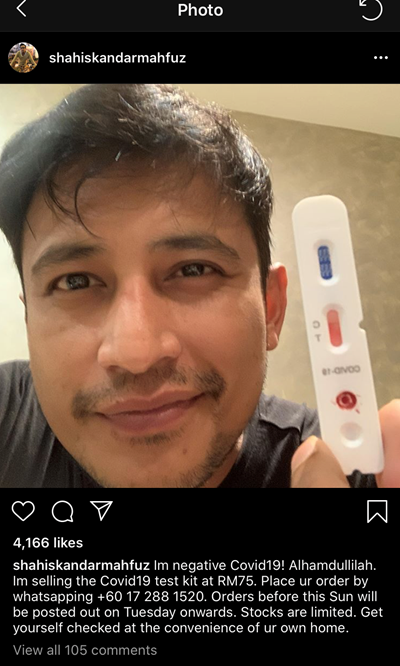
Pelakon Shah Iskandar nampaknya telah menarik perhatian netizen untuk dikecam kerana perlakuannya berkongsi kepada umum mengenai penjualan kit ujian pengesanan COVID-19 di Instagram miliknya, baru-baru ini.
Melalui postingnya itu, Shah, 38, turut memaklumkan bahawa dirinya negatif COVID-19 setelah menjalani ujian pengesanan menggunakan kit tersebut.
 Kredit foto: IG
"Saya negatif COVID-19! Alhamdullilah. Saya jual kit ujian COVID-19 pada harga RM75. Sila buat tempahan sebelum Ahad ini dan penghantaran akan dilakukan selepas Selasa. Lakukan pemeriksaan ke atas diri anda dengan hanya di rumah," tulisnya.
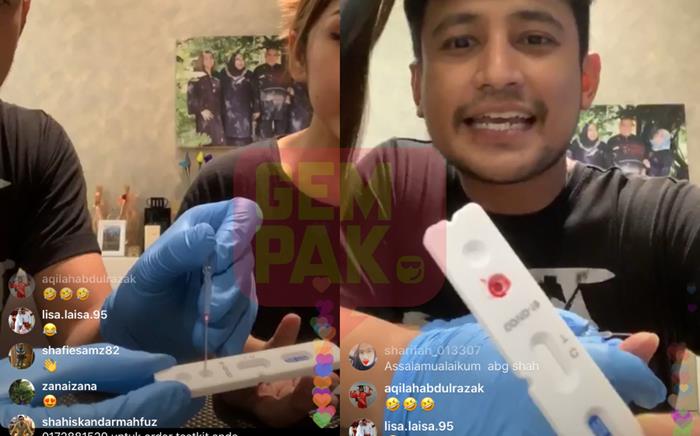
Malah, dia turut melakukan demonstrasi penggunaan kit tersebut menerusi sesi Live di Instagram.
Walau bagaimanapun, tindakannya itu kemudian mengundang pelbagai respons dari kalangan netizen.
 Kredit foto : Astro Gempak
Kredit foto : Astro Gempak
Rata-rate netizen mengkritik tindakan Shah yang menjual alat ujian pengesanan tersebut selain mempertikaikan perbuatannya kerana telah melanggar undang-undang.
Berikutan itu, Shah kemudian memadam posting tersebut dan membuat permohonan maaf secara terbuka di Live IG.
"Terima kasih kerana menyedarkan benda itu tak sepatutnya dijual," katanya.
Orang ramai yang menunjukan simptom COVID-19 diminta untuk terus ke hospital untuk mendapatkan rawatan. - CARI |
Rate
-
1
View Rating Log
-
|
|
|
|
|
|
|
|
|
|
|
|
Jangan ambil kesempatan atas kesusahan orang. Sekarang bukan masa nak buat duit |
|
|
|
|
|
|
|
|
|
|
|
|
Mcm pregnancy kit je dia guna tu. Hrmmm. |
|
|
|
|
|
|
|
|
|
|
|
|
Lencai mana ni. Makikan aje. |
|
|
|
|
|
|
|
|
|
|
|
|
Ade juga org beli ke?dah la mcm pregnancy test.euww |
|
|
|
|
|
|
|
|
|
|
|
Serupa pregnancy kit je. Mcm ni semua lelaki negatif {:give
Nk test pi hospital la.. |
|
|
|
|
|
|
|
|
|
|
|
|
Elok la dia pun dah mintak maaf.. |
|
|
|
|
|
|
|
|
|
|
|
|
Iols dgr ada home testing. Tepon doktor, pastu dia mai amik kat umah, rega RM700... |
|
|
|
|
|
|
|
|
|
|
|
Bukan testkits ni susah nak dapat ke? Cana heols dpt??
Btw, yg heran psl testkits tu roper preg testkits, so far iols nampak mmg mcm jenis cassette ni. Tp iols mmg heran cana heols dpt tu. Dahlah heols kan yg takde job sampai merayu dulu tu kan? |
|
|
|
|
|
|
|
|
|
|
|
Tinot7 replied at 23-3-2020 10:47 AM
Iols dgr ada home testing. Tepon doktor, pastu dia mai amik kat umah, rega RM700...
Ada... bleh g kt doctoroncall.com.my
Tp kna isi form dlu n tgk level of contact jugak...
Ini utk mudah kn org yg blk dr oversea, trus self kuarantin n xnk waste time n exposed keluar utk g screening kt hosp |
|
|
|
|
|
|
|
|
|
|
|
|
mohon PDRM tangkap mereka mereka yang ambil kesempatan time covid 19 |
|
|
|
|
|
|
|
|
|
|
|
Mentang2 la bodoh tuh free....sesuka hati tunjuk bodoh merata2... |
|
|
|
|
|
|
|
|
|
|
|
Kalau dia jual Di Singapore dah kena lock up mamat poyo ini  |
|
|
|
|
|
|
|
|
|
|
|
aechae replied at 23-3-2020 10:59 AM
Ada... bleh g kt doctoroncall.com.my
Tp kna isi form dlu n tgk level of contact jugak...
Ini utk ...
Ok tengkiu. Iols dgr ada spital dan makmal yg offer drive thru testing. Itu pun bagus utk org2 yg takut2... |
|
|
|
|
|
|
|
|
|
|
|
Tinot7 replied at 23-3-2020 11:53 AM
Ok tengkiu. Iols dgr ada spital dan makmal yg offer drive thru testing. Itu pun bagus utk org2 yg ...
Kna keluar rumah jugak kn klu cmtu...
Yg ni org dtg amek sample kt rumah cuma amek msa lh kot sbb mention ctu akn ada 1 day notice b4 dorg dtg...n result pn dlm 48-72hrs bru dpt |
|
|
|
|
|
|
|
|
|
|
|
Mmg mcm pregnancy test dah rupa..
Tapi nasib baik dia ni ada skit je cerdik minta maaf dan sedar x leh jual mende2 merepek lepas kena kecam..
Tak la mendongak je pertahankan diri mcm FL |
|
|
|
|
|
|
|
|
|
|
|
aechae replied at 23-3-2020 11:59 AM
Kna keluar rumah jugak kn klu cmtu...
Yg ni org dtg amek sample kt rumah cuma amek msa lh kot sbb ...
Hehe.. iols tanya2 ni, iols tak buat pun.. hihi... saje nak tau.. |
|
|
|
|
|
|
|
|
|
|
|
Tinot7 replied at 23-3-2020 12:05 PM
Hehe.. iols tanya2 ni, iols tak buat pun.. hihi... saje nak tau..
Kot klu ada duit lbih 700hengget mna tau saje nk test  |
|
|
|
|
|
|
|
|
|
|
|
Sial betul ko ni... muka hensem dah macam mat dadih... jual benda bodoh... masa dia promote pun aku dah pelik... biar betul mamat ni.....covid ni kena ambil sampel dalam mulut sampai belakang hidung... ngeri aku tgk |
|
|
|
|
|
|
|
|
|
| |
|